- Home
- Profile
- Services
- Calibration
- Consultation In Quality System
- Environmental Services
- Inspection Of Final Products
- Health, Safety And Risk Management
- Non Destructive Examination
- Preshipment Inspection
- Project Third Party Inspection
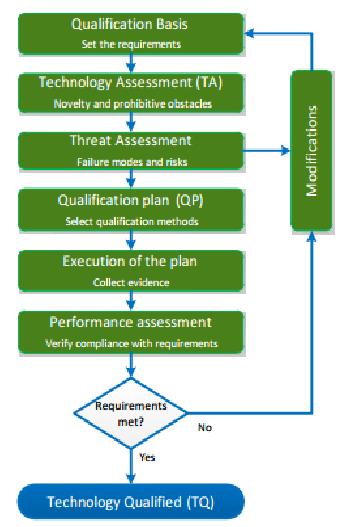
- Qualification Process
- Tally And Surveillance
- Temperature Mapping
- Validation Of Equipment
- Training
- Industries
- Accreditation
- Media Center
- Quotation
- Contact Us
- Blog